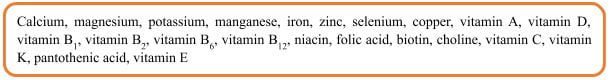Chinese consumer’s consumption of health food was far below that of developed countries in the past. However, presently the demand is increasing rapidly because of the aging aggravation, government encouragement, accelerated urbanization and other drivers.
China is recognized as the largest and fastest growing health food market which has huge potential. It is reported that the market scale of China’s health food industry will exceed 450 billion yuan in 2020. What’s more, Chinese consumers’ preferences for imported health food and Chinese government’s favorable policies on health products have brought great opportunity to foreign health food companies.
See Our Market Entry Services in Hong Kong & Mainland China
In 1996, Chinese government put up the first regulation on health food registration. After more than 20 years, the regulatory system has been increasingly improved. In accordance with the Food Safety Law of China (2015 version), companies who plan to import health food to Chinese market shall obtain the health food registration certificate or filing certificate. This is the first time that China implements a dual-track system of registration and filing.
If you are interested in developing health food business in China, you shall be aware of Chinese market and regulations. This article provides you with comprehensive information about the management of health food in China.
1. Introduction of Health Food
Health food refers to food products which claim the health function based on scientific basis, and have no acute, sub-acute or chronic hazards to human body. In Chinese industry, it is usually divided into two categories as following:
(i) Nutrition supplement:
Health food that provides vitamins and/or minerals but without providing energy or other active ingredients.
(ii) Functional health food:
Health food that is claimed with health function and has physiological effects on the human body. In accordance with current Chinese regulations, currently there are 27 health functions available for functional health food.
Companies who get the Chinese health food registration or filing certificate, could put the below “Blue Hat” LOGO in the packaging. And then customers know that this health food has approved.

Fig 1 Logo of approved health food in China (known as blue hat)
2. Huge Health Food Market in China
- Favorable Policies
I. In order to improve the health of the Chinese people, Chinese government generated the “Outline of Healthy China 2030” and “National Nutrition Program (2017-2030)”, which provide vast market opportunities for health food industry.
II. At present, China implements a dual-track system of health food registration and filing. Between them, health food filing policy is unprecedented. It simplifies the procedures of administrative examination, which helps to invigorate the market, and reduce the enterprise burden.
- Approval Status
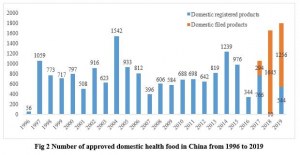
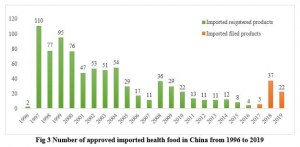
Since the implementation of health food registration in 1996, China has approved more than 17,000 registered health food products in total. Meanwhile, since the approval of the first filing product in 2017, more than 3000 filing products have been approved in the past three years. The simplified application requirements and review procedures accelerate the approval speed of filing products.
3. Compliance Procedures for Health Food Access to Chinese Market
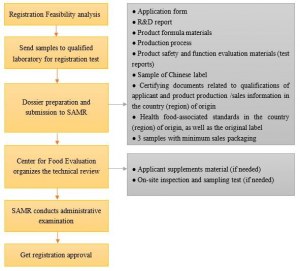
If you want to sell health food products in Chinese market, application of health food registration/filing is a prerequisite. You are required to submit the application dossiers to Chinese competent authority (SAMR, former CFDA) for review.
4. Current Regulation
On July 1st 2016, Administrative Measures on Health Food Registration and Filing come into force. This Measure is composed in accordance with the requirements of Food Safety Law of China, which stipulates the basic review procedures, competent authority, application dossier, labeling requirements, as well as the supervision measures of health food registration and filing in China.
I. China State Administration for Market Regulation (SAMR) is the competent authority for imported health food registration and filing.
II. Center for Food Evaluation, State Administration for Market Regulation is responsible for the technical review of imported health food application.
6. Application of Health Food Registration
- Scope of registration
All imported health food (excluded nutrition supplement of which the vitamins and (or) minerals meet the requirements in the Health Food Raw Materials Directory) shall apply for registration in China.
- Registration procedures
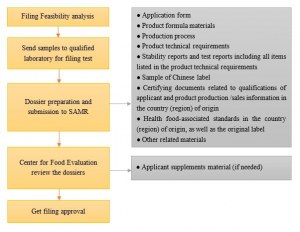
7. Application of Health Food Filing
- Scope of filing
The imported nutrition supplement of which the vitamins and (or) minerals meet the requirements in the Health Food Raw Materials Directory shall apply for filing in China.
Health Food Raw Materials Directory
Currently, 22 vitamins and minerals are listed in the Health Food Raw Materials Directory (the first batch), including:
- Filing procedures
8. Labeling Requirements
The Chinese authorities pay great attention to the supervision of health food labels. Main contents of the label shall be consistent with that of the registration/filing approval certificate. Moreover, on 20 August 2019, SAMR issued the Guideline on Warning Statements for Health Food Labeling, which has entered into force on January 1st, 2020. The following labeling requirements shall be focused on:
- It is forbidden to claim that the product is to prevent or treat any disease. And the warning statement of “health food is not drug, and cannot replace drug to treat disease” shall be printed on the prominent position of label.
- Production date and expiry date must be indicated in the order of “year/month/date”.
- Complaint hotline and hotline service time shall be marked. The font of complaint hotline shall be same with the font of health function.
Original article from CIRS China.
Are you interested in importing your products to Vietnam? Our Product Registration services can ensure a hassle-free process to expand your business.
About Us
InCorp Vietnam is a leading provider of global market entry services. We are part of InCorp group, a regional leader in corporate solutions, that encompasses 8 countries in Asia-Pacific, headquartered in Singapore. With over 1,100 legal experts serving over 15,000 Corporate Clients across the region, our expertise speaks for itself. We provide transparent legal consulting, setup, and advice based on local requirements to make your business perfectly fit into the market with healthy growth.
Don’t take our word for it. Read some reviews from some of our clients.